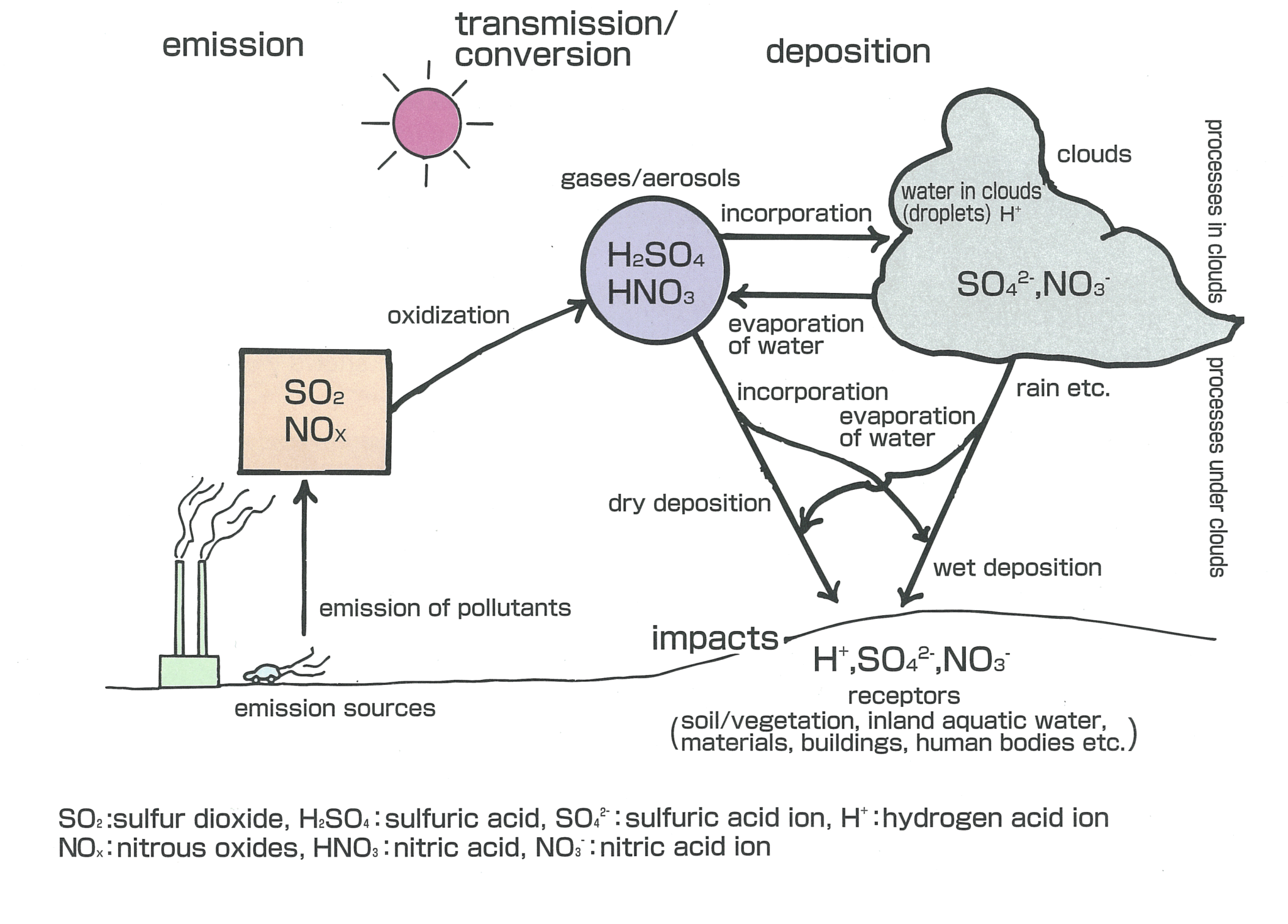
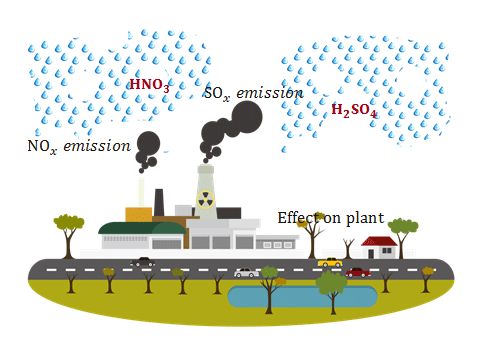
When sulfurous sulfuric and nitric acids in polluted air and rain react with the calcite in marble and limestone the calcite dissolves.
Is acid rain damaging a marble statue a physical change.
Although metal statues resist physical deterioration from acid rain better than stone they can develop discoloration and streaking.
Stone surface material may be lost all over or only in spots that are more reactive.
Anyway got off topic it is the acid in the rain reacting with the marble and producing gas possibly other stuff as well as i said can t remember thus it looks as though the marble is changing physically i m sure a google search of the make up of marble and acid rain and the possible reactions of the molecules will give you your answer.
No its a chemical change because the acid in the rain reacts with the copper in the statue having a reaction oxidizing it and turning it green.
In exposed areas of buildings and statues we see roughened surfaces removal of material and loss of carved details.
Acid rain damaging a marble statue is a physical change.
How does acid precipitation affect marble and limestone buildings.
When sulfurous sulfuric and nitric acids in polluted air react with the calcite in marble and limestone the calcite dissolves.
In exposed areas of buildings and statues we see roughened.
Acid rain has also attacked the chiseled words on some tombstones rendering them unreadable.
Over decades of exposure to acid rain the details of a statue can be lost slowly turning them into featureless blobs.