There are always two or more phases in a heterogeneous mixture where you can identify a region with properties that are distinct from those of another region even if they are the.
Is granite an example of heterogeneous mixture.
The ice and soda are in two distinct phases of matter solid.
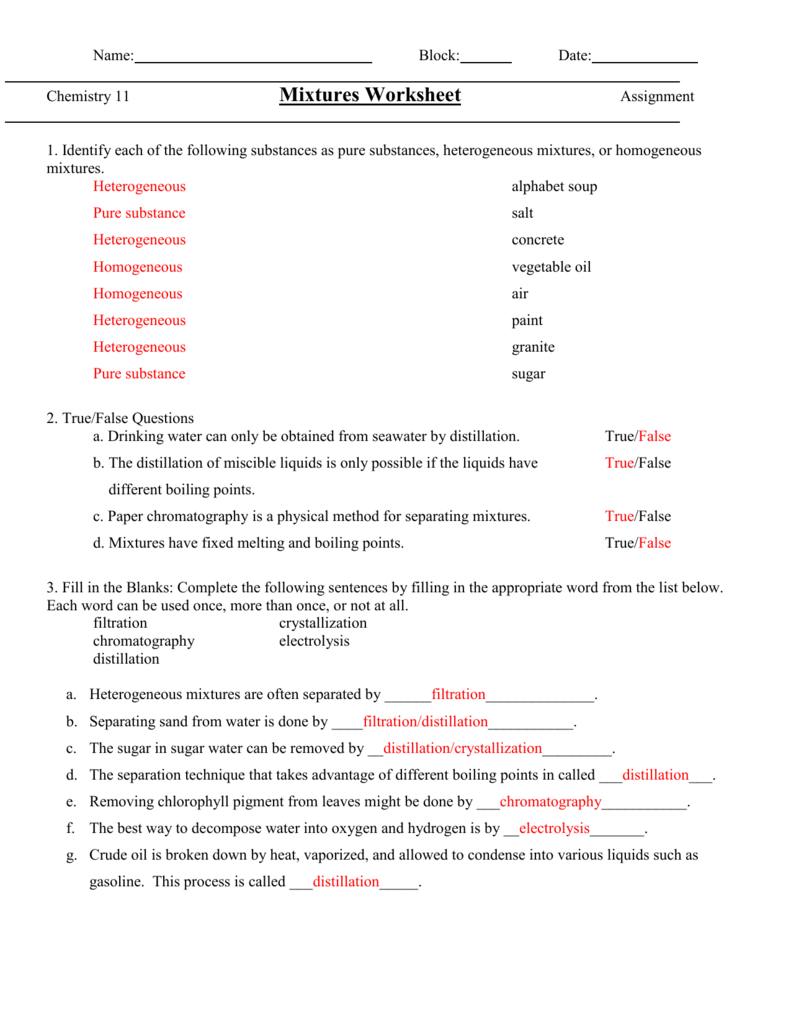
Examples of solutions include things like mud dust suspended in the air granite vinaigrette and sand.
The process of homogenization is what turns a heterogeneous mixture into a homogeneous mixture.
Examples include ice cubes in a drink sand and water and salt and oil.
Granite is a rock a non homogeneous mixture of minerals.
Cereal in the milk is an.
One of the most notable examples of homogenization is the process that produces homogenized milk.
A good example is a mixture of oil and water.
If you look closely you can identify tiny sugar crystals and particles of sand.
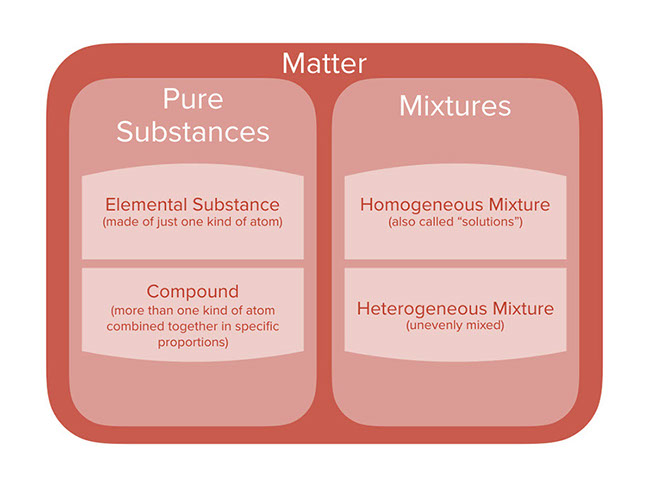
The mixture is a collection of substances which are formed by the chemical properties of more than one substance.
Concrete is a heterogeneous mixture of an aggregate.
Different samples from the mixture are not identical to each other.
The same goes for a salad of for example lettuce and tomato.
Is granite an element or a compound.
No granite is heterogeneous is granite a pure substance homogeneous mixture or heterogeneous mixture.
Ice cubes in cola form a heterogeneous mixture.
20 examples of heterogeneous mixtures september 28 2018 12 34 am in chemistry a mixture refers to the union of at least two substances in varying proportions without there being a chemical combination.
Sugar and sand form a heterogeneous mixture.
Which of the following items is not a compound.
7th grade science course textbook answers tagged examples of homogenous and heterogeneous mixtures ingilizce.
The liquid that is immiscible form heterogeneous mixtures.
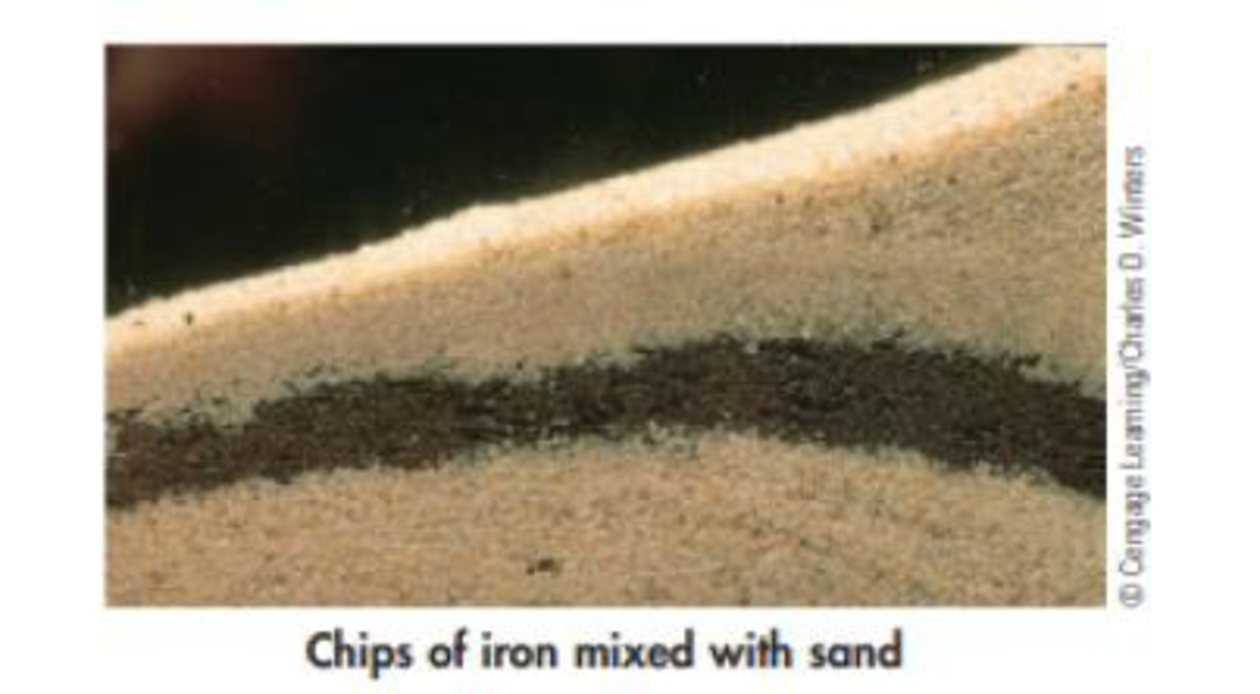
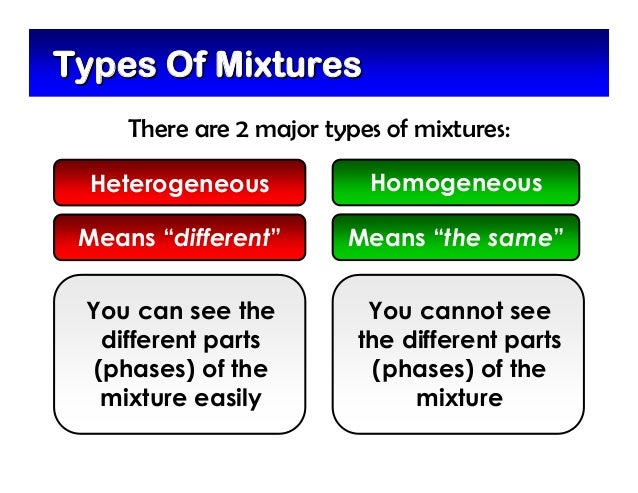
Mixtures in two or more phases are heterogeneous mixtures.
An example of a homogeneous mixture is.
Which of the following is true about homogeneous mixtures.
These are those in which the substances that make up the mixture eg oil and water can be distinguished at first glance.
Chemical solutions are usually homogeneous mixtures.
Examples of heterogeneous mixtures.
Since the substances do not combine.